[Published online Journal of Computer Chemistry, Japan Vol.19, 175-177, by J-STAGE]
<Title:> 珍しい塩基触媒による不斉Diels-Alder合成反応のMOシミュレーション解析
<Author(s):> 染川 賢一, 上田 岳彦, 吉留 俊史, 石川 岳志, 錦織 寿
<Corresponding author E-Mill:> somekw(at)voice.ocn.ne.jp
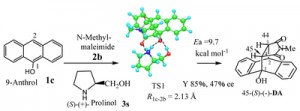
<Abstract:> The reaction process and steric situations of novel basic and chiral catalyst Diels-Alder reactions by Kagan et al. were speculated by IRC of PM7 simulation for the three molecules reactions clearly. The addition reactions of enolic dienes (1) with dienophiles (2) by amines (3) such as (S)-(+)-prolinol / (R)-(-)-prolinol proceeded via lower energy reaction complexes (RC) and transition states (TS) of two steps. The steric shapes by IRC (Figure 2 6) showed the clear interactions between the reaction points, and of OH with amine moieties in the 1 3, 1 3 2 and TS complexes, to give high stereoselective adducts. IRC of some reactions also guesses right the Michael reaction selectivity. The handy PM7 simulation is recommended for usual chemical growth.
<Keywords:> MOPAC2016-PM7, Diels-Alder reaction, Chiral and basic catalyst, Transition state, MO simulation, Reaction complex, IRC
<URL:> https://www.jstage.jst.go.jp/article/jccj/19/4/19_2021-0011/_article/-char/ja/
<Title:> 珍しい塩基触媒による不斉Diels-Alder合成反応のMOシミュレーション解析
<Author(s):> 染川 賢一, 上田 岳彦, 吉留 俊史, 石川 岳志, 錦織 寿
<Corresponding author E-Mill:> somekw(at)voice.ocn.ne.jp
<Abstract:> The reaction process and steric situations of novel basic and chiral catalyst Diels-Alder reactions by Kagan et al. were speculated by IRC of PM7 simulation for the three molecules reactions clearly. The addition reactions of enolic dienes (1) with dienophiles (2) by amines (3) such as (S)-(+)-prolinol / (R)-(-)-prolinol proceeded via lower energy reaction complexes (RC) and transition states (TS) of two steps. The steric shapes by IRC (Figure 2 6) showed the clear interactions between the reaction points, and of OH with amine moieties in the 1 3, 1 3 2 and TS complexes, to give high stereoselective adducts. IRC of some reactions also guesses right the Michael reaction selectivity. The handy PM7 simulation is recommended for usual chemical growth.
<Keywords:> MOPAC2016-PM7, Diels-Alder reaction, Chiral and basic catalyst, Transition state, MO simulation, Reaction complex, IRC
<URL:> https://www.jstage.jst.go.jp/article/jccj/19/4/19_2021-0011/_article/-char/ja/