[Published online Journal of Computer Chemistry, Japan Vol.20, 137-139, by J-STAGE]
<Title:> 第一原理計算によるカテキンの酸化還元電位の理論予測
<Author(s):> 段 練, 鷹野 優, 重田 育照
<Corresponding author E-Mill:> ytakano(at)hiroshima-cu.ac.jp
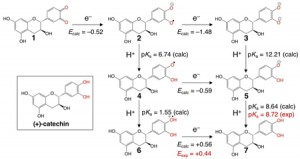
<Abstract:> Catechins are the main constituents in tea and have attracted attention due to their antioxidant properties. In this letter, the redox potential of single electron transfer and the pKa of proton transfer in (+)-catechin has been calculated at the B3LYP/6-31++G (d, p) level of theory with a recently developed scheme to evaluate the standard hydrogen electrode potential and redox potential. Our computational scheme reproduced the experimental redox potential and pKa value well.
<Keywords:> First-principles calculation, Catechin, Redox potential, Standard hydrogen electrode, p??i??K??/i????sub??a??/sub??
<URL:> https://www.jstage.jst.go.jp/article/jccj/20/4/20_2022-0002/_article/-char/ja/
<Title:> 第一原理計算によるカテキンの酸化還元電位の理論予測
<Author(s):> 段 練, 鷹野 優, 重田 育照
<Corresponding author E-Mill:> ytakano(at)hiroshima-cu.ac.jp
<Abstract:> Catechins are the main constituents in tea and have attracted attention due to their antioxidant properties. In this letter, the redox potential of single electron transfer and the pKa of proton transfer in (+)-catechin has been calculated at the B3LYP/6-31++G (d, p) level of theory with a recently developed scheme to evaluate the standard hydrogen electrode potential and redox potential. Our computational scheme reproduced the experimental redox potential and pKa value well.
<Keywords:> First-principles calculation, Catechin, Redox potential, Standard hydrogen electrode, p??i??K??/i????sub??a??/sub??
<URL:> https://www.jstage.jst.go.jp/article/jccj/20/4/20_2022-0002/_article/-char/ja/