[Published online Journal of Computer Chemistry, Japan Vol.24, 1-4, by J-STAGE]
<Title:> ジメチルスルホキシドの水和構造評価:有効フラグメントポテンシャル-分子動力学計算
<Author(s):> 石郷岡 知里, 黒木 菜保子
<Corresponding author E-Mill:> kuroki.nahoko(at)ocha.ac.jp
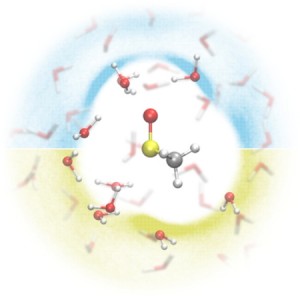
<Abstract:> Dimethyl sulfoxide (DMSO), widely used as a cryoprotectant, drug, and oxidant, contains a hydrophilic S=O and hydrophobic CH3 groups and is fully miscible with water in any ratio. In this study, intermolecular interactions in dilute aqueous DMSO solution were analyzed using effective fragment potential based molecular dynamics (EFP-MD) simulations. The analysis revealed that DMSO induced stable interactions with water molecules not only around its hydrophilic S=O group but also near the CH3 groups. The interaction lifetimes suggested that the hydrogen bonding network in the first hydration shell is directional.
<Keywords:> キーワードHydration, Fragmentation, Quantum chemistry, Molecular dynamics, Intermolecular interaction
<URL:> https://www.jstage.jst.go.jp/article/jccj/24/1/24_2024-0042/_article/-char/ja/
<Title:> ジメチルスルホキシドの水和構造評価:有効フラグメントポテンシャル-分子動力学計算
<Author(s):> 石郷岡 知里, 黒木 菜保子
<Corresponding author E-Mill:> kuroki.nahoko(at)ocha.ac.jp
<Abstract:> Dimethyl sulfoxide (DMSO), widely used as a cryoprotectant, drug, and oxidant, contains a hydrophilic S=O and hydrophobic CH3 groups and is fully miscible with water in any ratio. In this study, intermolecular interactions in dilute aqueous DMSO solution were analyzed using effective fragment potential based molecular dynamics (EFP-MD) simulations. The analysis revealed that DMSO induced stable interactions with water molecules not only around its hydrophilic S=O group but also near the CH3 groups. The interaction lifetimes suggested that the hydrogen bonding network in the first hydration shell is directional.
<Keywords:> キーワードHydration, Fragmentation, Quantum chemistry, Molecular dynamics, Intermolecular interaction
<URL:> https://www.jstage.jst.go.jp/article/jccj/24/1/24_2024-0042/_article/-char/ja/