[Published online Journal of Computer Chemistry, Japan Vol.16, 93-95, by J-STAGE]
<Title:> Quantum Chemical Study on the Multi-Electron Transfer of Keggin-Type Polyoxotungstate Anions: The Relation of Redox Potentials to the Bond Valence of 4-O-W
<Author(s):> Aki TAKAZAKI, Kazuo EDA, Toshiyuki OSAKAI, Takahito NAKAJIMA
<Corresponding author E-Mill:> 148s231s(at)stu.kobe-u.ac.jp
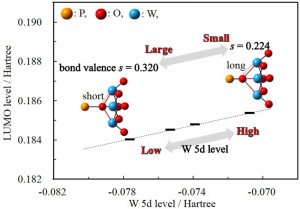
<Abstract:> By selectively investigating the effect of the bond valence using the hypothetical [(PO4)W12O36]3 species having various bond valences, we could clearly reveal the origin of the linear dependence of the LUMO energy (or the redox potential) on the bond valence. The LUMO of the Keggin-type polyoxotungstates mainly consists of W 5d. The energy of W 5d as well as of the LUMO goes down as the bond valence becomes large (i.e., as the net electron population on W decreases due to the electron-withdrawing effect of the μ4-O atoms). This is the origin of the linear dependence of LUMO energy on the bond valence.
<Keywords:> Multi-electron transfer, Polyoxometalate, Polyoxotungstate, DFT, LUMO
<URL:> https://www.jstage.jst.go.jp/article/jccj/16/4/16_2017-0029/_article/-char/ja/
<Title:> Quantum Chemical Study on the Multi-Electron Transfer of Keggin-Type Polyoxotungstate Anions: The Relation of Redox Potentials to the Bond Valence of 4-O-W
<Author(s):> Aki TAKAZAKI, Kazuo EDA, Toshiyuki OSAKAI, Takahito NAKAJIMA
<Corresponding author E-Mill:> 148s231s(at)stu.kobe-u.ac.jp
<Abstract:> By selectively investigating the effect of the bond valence using the hypothetical [(PO4)W12O36]3 species having various bond valences, we could clearly reveal the origin of the linear dependence of the LUMO energy (or the redox potential) on the bond valence. The LUMO of the Keggin-type polyoxotungstates mainly consists of W 5d. The energy of W 5d as well as of the LUMO goes down as the bond valence becomes large (i.e., as the net electron population on W decreases due to the electron-withdrawing effect of the μ4-O atoms). This is the origin of the linear dependence of LUMO energy on the bond valence.
<Keywords:> Multi-electron transfer, Polyoxometalate, Polyoxotungstate, DFT, LUMO
<URL:> https://www.jstage.jst.go.jp/article/jccj/16/4/16_2017-0029/_article/-char/ja/