[Published online Journal of Computer Chemistry, Japan -International Edition Vol.3, -, by J-STAGE]
<Title:> Calculation of the Melting Entropy of Argon at Constant Volume Using Molecular Dynamics
<Author(s):> Yosuke KATAOKA
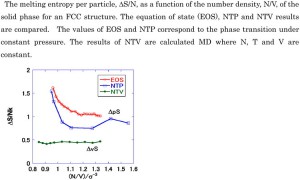
<Abstract:> The melting entropy values of argon at constant volume (ΔvS) as a function of density were calculated using molecular dynamics simulations. These calculations employed cells with N = 864 molecules. The resulting entropy of melting per particle, ΔvS/N, was found to be essentially constant (with some slight effect of the crystal form) and nearly equal to 0.5 k, where k is the Boltzmann constant. The melting entropy values at constant pressure (ΔpS) were also determined and it was observed that ΔpS > ΔvS. In addition, variations in the vibrational frequency in the liquid were compared with fluctuations in the solid near the melting point as a means of further investigating the melting entropy.
<Keywords:> Melting entropy at constant volume, Argon, Molecular dynamics, Fluctuation of vibrational frequency
<URL:> https://www.jstage.jst.go.jp/article/jccjie/3/0/3_2017-0021/_html
<Title:> Calculation of the Melting Entropy of Argon at Constant Volume Using Molecular Dynamics
<Author(s):> Yosuke KATAOKA
<Abstract:> The melting entropy values of argon at constant volume (ΔvS) as a function of density were calculated using molecular dynamics simulations. These calculations employed cells with N = 864 molecules. The resulting entropy of melting per particle, ΔvS/N, was found to be essentially constant (with some slight effect of the crystal form) and nearly equal to 0.5 k, where k is the Boltzmann constant. The melting entropy values at constant pressure (ΔpS) were also determined and it was observed that ΔpS > ΔvS. In addition, variations in the vibrational frequency in the liquid were compared with fluctuations in the solid near the melting point as a means of further investigating the melting entropy.
<Keywords:> Melting entropy at constant volume, Argon, Molecular dynamics, Fluctuation of vibrational frequency
<URL:> https://www.jstage.jst.go.jp/article/jccjie/3/0/3_2017-0021/_html