[Published online Journal of Computer Chemistry, Japan Vol.16, 131-132, by J-STAGE]
<Title:> 計算化学手法によるフラーレンの位置選択的反応の解析
<Author(s):> 伊熊 直彦
<Corresponding author E-Mill:> ikuma(at)chem.eng.osaka-u.ac.jp
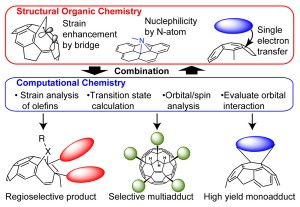
<Abstract:> Organic reactions with fullerene realize functional nanomaterials, although selective functionalization is still challenging because of many equivalent olefins causing various regioisomers. Thus, the improvement of reactivity and regioselectivity of C60 reaction has been realized by structural organic chemistry such as enhancement of strain, introduction of heteroatom, and nucleophilic additoin via single eletron transfer (Figure 1 Figure 1. Concept of the activation of C60 to obtain regioselective adducts. ). This paper presents the theoretical clarification for the reactivity and selectivity of these activated reaction conditions.
<Keywords:> Fullerene, Regioselectivity, Strain energy, Ambident basicity, Single elecron transfer
<URL:> https://www.jstage.jst.go.jp/article/jccj/16/5/16_2017-0054/_html/-char/ja/
<Title:> 計算化学手法によるフラーレンの位置選択的反応の解析
<Author(s):> 伊熊 直彦
<Corresponding author E-Mill:> ikuma(at)chem.eng.osaka-u.ac.jp
<Abstract:> Organic reactions with fullerene realize functional nanomaterials, although selective functionalization is still challenging because of many equivalent olefins causing various regioisomers. Thus, the improvement of reactivity and regioselectivity of C60 reaction has been realized by structural organic chemistry such as enhancement of strain, introduction of heteroatom, and nucleophilic additoin via single eletron transfer (Figure 1 Figure 1. Concept of the activation of C60 to obtain regioselective adducts. ). This paper presents the theoretical clarification for the reactivity and selectivity of these activated reaction conditions.
<Keywords:> Fullerene, Regioselectivity, Strain energy, Ambident basicity, Single elecron transfer
<URL:> https://www.jstage.jst.go.jp/article/jccj/16/5/16_2017-0054/_html/-char/ja/