[Published online Journal of Computer Chemistry, Japan Vol.18, 136-138, by J-STAGE]
<Title:> カチオン性イリジウム触媒を用いた均一系触媒反応における相対論効果
<Author(s):> 髙島 千波, 五十幡 康弘, 栗田 久樹, 高野 秀明, 柴田 高範, 中井 浩巳
<Corresponding author E-Mill:> nakai(at)waseda.jp
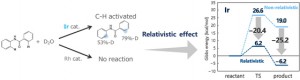
<Abstract:> A cationic Ir complex was reported to show specific catalytic activity in C-H bond activation reaction of benzanilides. The present study examined reaction energy profiles of the C-H bond activation with Ir and Rh catalysts based on non-relativistic and relativistic quantum chemical calculations. We found that the relativistic effect is essential to demonstrate the difference in the catalytic activity. In particular, the activation of the d orbital of Ir, which is caused by the s- and p- orbital contraction followed by the self-consistent d-orbital expansion, leads to stabilization of the transition state and product of the C-H bond activation.
<Keywords:> C-H Bond activation, Transition metal catalyst, Relativistic effect, Oxidative addition, Iridium
<URL:> https://www.jstage.jst.go.jp/article/jccj/18/3/18_2019-0021/_article/-char/ja/