[Published online Journal of Computer Chemistry, Japan Vol.18, 233-235, by J-STAGE]
<Title:> アシルピリジニウムカチオンの安定性および反応性の評価
<Author(s):> 柴田 裕介, 林 慶浩, 川内 進
<Corresponding author E-Mill:> kawauchi.s.aa(at)m.titech.ac.jp
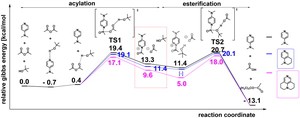
<Abstract:> Among ester synthesis methods, there is a method using a pyridine derivative as a catalyst, and the esterification reaction rate may be increased through a highly active acylpyridinium cation intermediate. It has been reported that the stability of acylpyridinium cations correlates with the reaction rate of esterification. In this study, we try to clarify the reason for the correlation between the reaction rate and the stability, then we investigate the relationship between the stability and structures of acylpyridinium cations. As a result, we found that the stability largely depends on the electronic donor property of the substituents, and acylpyridinium cation may act as an effective catalyst for esterification when the substituents have strong donor property at positions 3, 4, 5 of the pyridine ring.
<Keywords:> DFT, NBO deletion, esterification, pyridine derivatives, acylpyridinium cations
<URL:> https://www.jstage.jst.go.jp/article/jccj/18/5/18_2019-0049/_article/-char/ja/