[Published online Journal of Computer Chemistry, Japan Vol.17, 102-109, by J-STAGE]
<Title:> フラグメント分子軌道(FMO)法を用いた散逸粒子動力学シミュレーションのための有効相互作用パラメータ算出の自動化フレームワーク
<Author(s):> 奥脇 弘次, 土居 英男, 望月 祐志
<Corresponding author E-Mill:> okuwaki(at)rikkyo.ac.jp
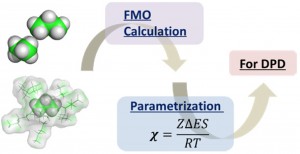
<Abstract:> 近年,用途に応じて目的の機能を有した材料開発の要求が高まっており,大規模な構造予測のためのシミュレーションが注目を浴びている.私達は,今回,散逸粒子動力学(DPD: Dissipative Particle Dynamics)シミュレーションを例に,有効相互作用パラメータ(χパラメータと呼ばれる) をフラグメント分子軌道(FMO)法から算定するためのフレームワークとワークフローシステムを開発した.FMO計算は非経験的であるため,高分子系素材に関わるほとんどの有機化合物に対して使うことが可能であり,χパラメータの算定に対して汎用性を持つと考えられる.この報告では,本システム(FCEWS: FMO-based Chi-parameter Evaluation Workflow System)の開発の目的,理論的な背景,ならびにソフトウェアについて解説する.
<Keywords:> Dissipative particle dynamics, DPD, fragment molecular orbital, FMO, chi-parameter
<URL:> https://www.jstage.jst.go.jp/article/jccj/17/2/17_2017-0048/_article/-char/ja/