[Published online Journal of Computer Chemistry, Japan Vol.20, 150-154, by J-STAGE]
<Title:> Effect of Pore Size of Carbon Support on Electrode Reaction Activity of Catalyst Layer in Polymer Electrolyte Fuel Cell: Reactive Molecular Dynamics Simulations
<Author(s):> Tetsuya NAKAMURA, Riku OTSUKI, Shuichi UEHARA, Yuta ASANO, Qian CHEN, Yusuke OOTANI, Nobuki OZAWA, Momoji KUBO
<Corresponding author E-Mill:> momoji(at)tohoku.ac.jp
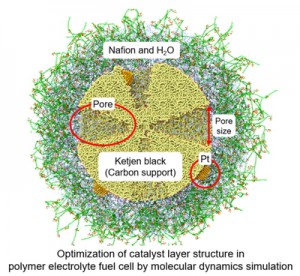
<Abstract:> For large output of polymer electrolyte fuel cells (PEFCs), the electrode reaction activity of the catalyst layer (CL) consisting of carbon supports, Pt nanoparticles, Nafion chains, and water should be improved. Experimentally, it is reported that when Ketjen black (KB) with meso pores is used as the carbon support, the output of PEFC increases and that the pore size of the KB support affects the electrode reaction activity of the Pt nanoparticles. Therefore, in the present study, to clarify the effect of pore size on the electrode reaction activity of the Pt nanoparticles, we constructed catalyst particle (CP) models in which the Pt nanoparticles are supported and Nafion chains are coated on the KB model and investigated the CP structures with a different pore size of the KB support by reactive molecular dynamics method. Regardless of the pore size, the Pt nanoparticles on the exterior of the pore are fully covered with the Nafion chains and the Pt nanoparticles in the interior of the pore are not covered with the Nafion chains. This result suggests that the Pt nanoparticles in the interior of the pore show high oxygen transport property that does not depend on the pore size. Furthermore, we evaluated the connectivity of the Nafion chains to H2O molecules absorbed on the Pt nanoparticles on the exterior and in the interior of the pores because the Nafion chains conduct the protons to the H2O molecules on the Pt nanoparticles. As the pore size increases, more Nafion chains penetrate the interior of the pore and contact with H2O molecules on the Pt nanoparticles, because more Nafion chains are vertically distributed above the larger pore. Finally, these results propose that both high oxygen transport property and high electrode reaction activity are achieved over the Pt nanoparticles in the interior of the large pore of the KB support because the oxygen diffusion in the pore is not blocked by the Nafion chains and the large pore size promotes the formation of a proton conducting path composed of the Nafion chains, H2O, and Pt nanoparticles.
<Keywords:> Reactive Molecular Dynamics, Polymer Electrolyte Fuel Cell, Catalyst Layer, Ketjen Black, Reaction Activity
<URL:> https://www.jstage.jst.go.jp/article/jccj/20/4/20_2022-0008/_article/-char/ja/
<Title:> Effect of Pore Size of Carbon Support on Electrode Reaction Activity of Catalyst Layer in Polymer Electrolyte Fuel Cell: Reactive Molecular Dynamics Simulations
<Author(s):> Tetsuya NAKAMURA, Riku OTSUKI, Shuichi UEHARA, Yuta ASANO, Qian CHEN, Yusuke OOTANI, Nobuki OZAWA, Momoji KUBO
<Corresponding author E-Mill:> momoji(at)tohoku.ac.jp
<Abstract:> For large output of polymer electrolyte fuel cells (PEFCs), the electrode reaction activity of the catalyst layer (CL) consisting of carbon supports, Pt nanoparticles, Nafion chains, and water should be improved. Experimentally, it is reported that when Ketjen black (KB) with meso pores is used as the carbon support, the output of PEFC increases and that the pore size of the KB support affects the electrode reaction activity of the Pt nanoparticles. Therefore, in the present study, to clarify the effect of pore size on the electrode reaction activity of the Pt nanoparticles, we constructed catalyst particle (CP) models in which the Pt nanoparticles are supported and Nafion chains are coated on the KB model and investigated the CP structures with a different pore size of the KB support by reactive molecular dynamics method. Regardless of the pore size, the Pt nanoparticles on the exterior of the pore are fully covered with the Nafion chains and the Pt nanoparticles in the interior of the pore are not covered with the Nafion chains. This result suggests that the Pt nanoparticles in the interior of the pore show high oxygen transport property that does not depend on the pore size. Furthermore, we evaluated the connectivity of the Nafion chains to H2O molecules absorbed on the Pt nanoparticles on the exterior and in the interior of the pores because the Nafion chains conduct the protons to the H2O molecules on the Pt nanoparticles. As the pore size increases, more Nafion chains penetrate the interior of the pore and contact with H2O molecules on the Pt nanoparticles, because more Nafion chains are vertically distributed above the larger pore. Finally, these results propose that both high oxygen transport property and high electrode reaction activity are achieved over the Pt nanoparticles in the interior of the large pore of the KB support because the oxygen diffusion in the pore is not blocked by the Nafion chains and the large pore size promotes the formation of a proton conducting path composed of the Nafion chains, H2O, and Pt nanoparticles.
<Keywords:> Reactive Molecular Dynamics, Polymer Electrolyte Fuel Cell, Catalyst Layer, Ketjen Black, Reaction Activity
<URL:> https://www.jstage.jst.go.jp/article/jccj/20/4/20_2022-0008/_article/-char/ja/