[Published online Journal of Computer Chemistry, Japan -International Edition Vol.8, -, by J-STAGE]
<Title:> A Tautomerization Software Based on Lewis Structures and Reaction Mechanisms
<Author(s):> Ming YU
<Corresponding author E-Mill:> yuming086(at)gmail.com
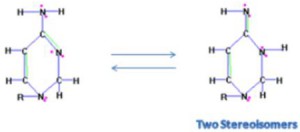
<Abstract:> A new method is proposed to translate molecule Lewis structures to machine-readable format data and a software based on Lewis structures has been developed to simulate the practice of chemists writing tautomerization mechanisms. For a tautomeric compound, from one Lewis formula entered by users, the software can generate its other equivalent tautomer (s), which share a common tautomerization intermediate stabilized by resonance. Not only atom balance but also electron balance are guaranteed in the tautomerism representation. The stereochemistry changes caused by tautomeric equilibrium can be tracked too. All the stereoisomers for the compounds containing tetrahedral carbon stereocenters and double bond stereocenters can be identified.
<Keywords:> Tautomerization, Software, Stereochemistry, Lewis structure, Mechanism
<URL:> https://www.jstage.jst.go.jp/article/jccjie/8/0/8_2021-0050/_html
<Title:> A Tautomerization Software Based on Lewis Structures and Reaction Mechanisms
<Author(s):> Ming YU
<Corresponding author E-Mill:> yuming086(at)gmail.com
<Abstract:> A new method is proposed to translate molecule Lewis structures to machine-readable format data and a software based on Lewis structures has been developed to simulate the practice of chemists writing tautomerization mechanisms. For a tautomeric compound, from one Lewis formula entered by users, the software can generate its other equivalent tautomer (s), which share a common tautomerization intermediate stabilized by resonance. Not only atom balance but also electron balance are guaranteed in the tautomerism representation. The stereochemistry changes caused by tautomeric equilibrium can be tracked too. All the stereoisomers for the compounds containing tetrahedral carbon stereocenters and double bond stereocenters can be identified.
<Keywords:> Tautomerization, Software, Stereochemistry, Lewis structure, Mechanism
<URL:> https://www.jstage.jst.go.jp/article/jccjie/8/0/8_2021-0050/_html