[Published online Journal of Computer Chemistry, Japan Vol.21, 55-57, by J-STAGE]
<Title:> Structural Symmetry and Spin Multiplicity of Sumanene Derivative Radical Molecules
<Author(s):> Yuika BABA, Hidehiro SAKURAI, Azusa MURAOKA
<Corresponding author E-Mill:> muraokaa(at)fc.jwu.ac.jp
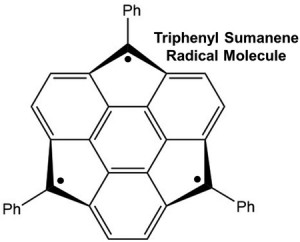
<Abstract:> π-conjugated carbon molecules such as fullerenes and carbon nanotubes have attracted great interest because of their potential applications as electronic materials and catalysts. Among these molecular frameworks, fullerene substructures of sumanene molecules with C3v symmetry are expected to form vertical columnar crystal structures and exhibit a range of conduction properties. Various syntheses of molecules with extended π-conjugation at the benzyl position of sumanene have now been reported. However, there are no examples of open-shell systems with extended sumanene features. In this study, we use the results of DFT calculations to discuss the relationship between molecular conformational symmetry and spin multiplicity in a new, extended open-shell system of sumanenes by adding a phenyl group at the benzyl position.
<Keywords:> Sumanene derivatives, Radical molecules, Molecular symmetry, Spin multiplicity, DFT calculations
<URL:> https://www.jstage.jst.go.jp/article/jccj/21/2/21_2022-0033/_article/-char/ja/
<Title:> Structural Symmetry and Spin Multiplicity of Sumanene Derivative Radical Molecules
<Author(s):> Yuika BABA, Hidehiro SAKURAI, Azusa MURAOKA
<Corresponding author E-Mill:> muraokaa(at)fc.jwu.ac.jp
<Abstract:> π-conjugated carbon molecules such as fullerenes and carbon nanotubes have attracted great interest because of their potential applications as electronic materials and catalysts. Among these molecular frameworks, fullerene substructures of sumanene molecules with C3v symmetry are expected to form vertical columnar crystal structures and exhibit a range of conduction properties. Various syntheses of molecules with extended π-conjugation at the benzyl position of sumanene have now been reported. However, there are no examples of open-shell systems with extended sumanene features. In this study, we use the results of DFT calculations to discuss the relationship between molecular conformational symmetry and spin multiplicity in a new, extended open-shell system of sumanenes by adding a phenyl group at the benzyl position.
<Keywords:> Sumanene derivatives, Radical molecules, Molecular symmetry, Spin multiplicity, DFT calculations
<URL:> https://www.jstage.jst.go.jp/article/jccj/21/2/21_2022-0033/_article/-char/ja/