[Published online Journal of Computer Chemistry, Japan -International Edition Vol.4, -, by J-STAGE]
<Title:> Determining the Three-phase Equilibrium Diagrams for Water, Oxygen, Propane and Lithium Using van der Waals Equations of State
<Author(s):> Yosuke KATAOKA
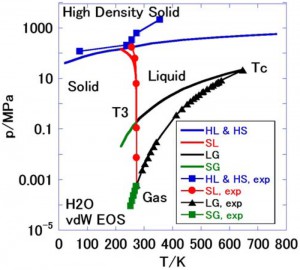
<Abstract:> In the present work, phase diagrams in (pressure, temperature) space were calculated for water, oxygen, propane and lithium, employing van der Waals (vdW) equations of state, similar to an approach previously employed for argon. These calculations allowed the triple point temperatures to be obtained. The experimentally-determined triple point pressure values (p3) normalized by the critical point pressure (pc) (that is, p3/pc) are known to exhibit a significant correlation with the normalized triple point temperature (T3/Tc), and the present results reproduce this correlation qualitatively. This work also demonstrated that the low-density solid, high-density solid, liquid and gas-phases of water produce a phase diagram similar to that of the ice-water system.
<Keywords:> Three-phase equilibrium, Van der Waals equation of state, Vapor pressure, Sublimation pressure, Melting curve, Water, Oxygen, Propane, Lithium
<URL:> https://www.jstage.jst.go.jp/article/jccjie/4/0/4_2017-0042/_html