[Published online Journal of Computer Chemistry, Japan Vol.17, 217-218, by J-STAGE]
<Title:> Rh触媒による3-フェニルチオフェンとスチレンのカップリング反応における位置選択性の理論的研究
<Author(s):> 林 慶浩, 佐藤 哲也, 三浦 雅博, 川内 進
<Corresponding author E-Mill:> skawauch(at)polymer.titech.ac.jp
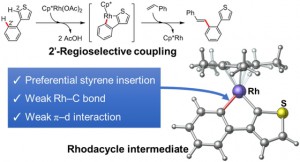
<Abstract:> Regioselectivity in the Rh-catalyzed coupling reaction of 3-phenylthiophene with styrene was investigated by quantum chemistry calculations. The coupling reaction experimentally reported that styrene is selectively coupled to the phenyl group of the phenylthiophene. Results of the reaction path search and the natural bond orbital analysis indicate that the coupling position is determined by the preference of the styrene insertion into the 5-membered rhodacycle intermediate, which is produced by the double C H bond cleavage of 3-phenylthiophene in presence of the Rh-catalyst.
<Keywords:> カップリング反応, C-H官能基化, ロジウム触媒, 反応機構, DFT計算
<URL:>
https://www.jstage.jst.go.jp/article/jccj/17/5/17_2018-0063/_article/-char/ja/