[Published online Journal of Computer Chemistry, Japan Vol.15, 192-198, by J-STAGE]
<Title:> Ionic Hydrogen Bonding Vibration in OH–(H2O)2-4
<Author(s):> Masato MORITA, Kaito TAKAHASHI
<Corresponding author E-Mill:> kt(at)gate.sinica.edu.tw
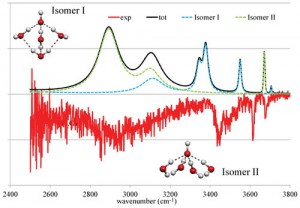
<Abstract:> Focusing on the OH–(H2O)2 4 clusters, we have theoretically studied the strongly red shifted ionic hydrogen bond (IHB) OH stretching vibration of water molecules directly bound to the hydroxide. Our calculations show that a systematic blue shift of the IHB OH peak is observed with the increase in the number of water molecules in the first solvation shell. Furthermore, we showed that the vibrational signature of the four coordinated hydroxide for OH–(H2O)4 will be observed in the 2800 3200 cm-1 range.
<Keywords:> Anharmonic proton vibration, Hydroxide water cluster, Hydrogen bonding, Anion hydration, Quantum chemistry
<URL:> https://www.jstage.jst.go.jp/article/jccj/15/5/15_2016-0012/_article/-char/ja/
<Title:> Ionic Hydrogen Bonding Vibration in OH–(H2O)2-4
<Author(s):> Masato MORITA, Kaito TAKAHASHI
<Corresponding author E-Mill:> kt(at)gate.sinica.edu.tw
<Abstract:> Focusing on the OH–(H2O)2 4 clusters, we have theoretically studied the strongly red shifted ionic hydrogen bond (IHB) OH stretching vibration of water molecules directly bound to the hydroxide. Our calculations show that a systematic blue shift of the IHB OH peak is observed with the increase in the number of water molecules in the first solvation shell. Furthermore, we showed that the vibrational signature of the four coordinated hydroxide for OH–(H2O)4 will be observed in the 2800 3200 cm-1 range.
<Keywords:> Anharmonic proton vibration, Hydroxide water cluster, Hydrogen bonding, Anion hydration, Quantum chemistry
<URL:> https://www.jstage.jst.go.jp/article/jccj/15/5/15_2016-0012/_article/-char/ja/