[Published online Journal of Computer Chemistry, Japan Vol.18, 224-226, by J-STAGE]
<Title:> 芳香族・キノイド性およびドナー・アクセプター性を用いた狭バンドギャップπ共役系高分子予測モデルの構築
<Author(s):> 林 慶浩, 川内 進
<Corresponding author E-Mill:> hayashi.y.ah(at)m.titech.ac.jp
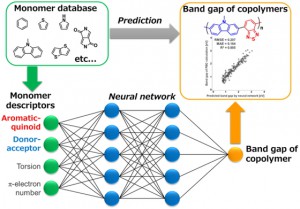
<Abstract:> A band gap prediction model of π-conjugated polymers was constructed using aromatic/quinoid, donor/acceptor, and torsion properties to predict quantitatively the band gap of π-conjugated polymers from properties of monomers. Quinoid stabilization energy (QSE), energy difference between HOMO of donor and LUMO of acceptor, torsion angle in homo-dimer of monomers were used as descriptors of aromatic/quinoid, donor/acceptor, and torsion properties. The neural network, which was constructed by 2 hidden layers with 5 neurons per layer, quantitatively predicts (RMSD = 0.207 eV, R2 = 0.885) the band gap of the π-conjugated polymers from descriptors of monomers.
<Keywords:> 高分子, バンドギャップ, 設計指標, DFT計算, 機械学習
<URL:> https://www.jstage.jst.go.jp/article/jccj/18/5/18_2019-0033/_article/-char/ja/